
Compressed and liquefied gases are routinely used in laboratories, shops and various other operations at the University. Compressed gas is a generic term used for describing compressed gases, liquefied compressed gases, refrigerated liquefied gases (cryogenic fluids) and dissolved gases.
Gas cylinders and Dewars containing compressed gases must be handled with caution at all times, and especially during transport. Assume all cylinders and Dewars contain gas under pressure. Treat all gases as hazardous chemicals.
Compressed Gas Cylinders Safety Guidelines
Refer to the Compressed Gas Cylinders Safety Guidelines for detailed information on hazards, safe use, storage, transport, disposal, leaks and emergencies. The guidelines apply to all University personnel and students who handle or use compressed or liquefied gases or systems.
All cylinders should bear a cylinder status tag stating one of the three conditions: full, in-service (part full) or empty.

To dispose of your compressed gas cylinders, ask yourself these questions:
Do you own the cylinder or rent it?
RENT: For example, when you procure a gas cylinder from a vendor such as Linde, you buy the gas but rent the cylinder (pay demurrage).
When you are done with the cylinder, you contact the company listed on the cylinder and they will pick it up.
OWN: If you own the cylinder, skip to the next question.
Is the cylinder empty?
YES: If the cylinder is empty, depending upon original contents, the valve can be removed and the cylinder disposed of as scrap metal.
To recycle empty, vented, and de-valved cylinders as scrap metal, remove or deface all labels, write “empty” on it, and contact UW Recycling to arrange a pickup.
NO: If the cylinder is not empty, but only contains common atmospheric gases such as nitrogen, it may be safely vented and emptied, de-valved and disposed of as scrap metal.
Contact EH&S Environmental Programs for guidance on removing the valve on your particular cylinder. If you are not able or willing to de-valve your cylinder, we can do it for you. There may be a small disposal fee charged for each cylinder. Please provide a budget number with your request.
All other non-rented, non-empty cylinders can be disposed of by submitting an chemical collection request form.
Users of compressed gas cylinders must be familiar with procedures for responding in an emergency. Standard operating procedures (SOPs) for using compressed gases must include possible incident scenarios with appropriate responses.
In the event of a large gas release or other incident, activate the following emergency procedures:
- Evacuate the area, securing entrances and aiding others on the way out.
- Activate building and area fire alarms (or chemical safety alarms if applicable).
- Call 9-1-1.
- Report the incident to a supervisor.
- Follow the EH&S exposure response procedures if potentially exposed to hazardous materials.
- Provide emergency response officials with details of the problem upon their arrival.
Refer to the Compressed Gas Cylinders Safety Guidelines for response procedures for a small gas leak.
Personnel are required to report all incidents to EH&S promptly via the Online Accident Report System (OARS).
The use of manifolds, piping, valves, and/or regulators must follow the Compressed Gas Cylinders Safety Guidelines.
Gases are classified in different ways according to definitions by different regulations including Department of Transportation (DOT) and Fire Code regulations as well as by classification systems such as the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). These classifications are different and overlap in some ways.
Users need to know the hazard classifications of the gases for their SOP(s), training, to plan for storage, to plan for any transport needed, and for addressing fire permits and hazardous materials quantities.
The Fire Code definitions for compressed gases are listed in the Definitions section on this page.
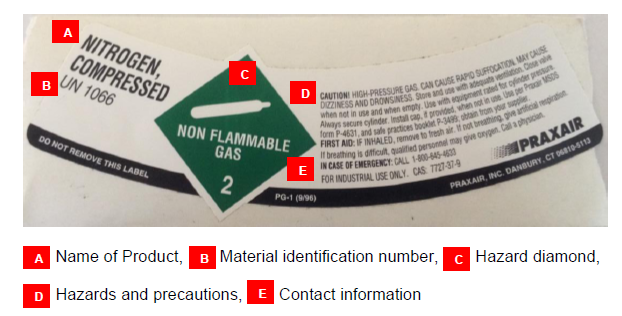
Cylinders stored and used at a University location must be clearly labeled according to U.S. Department of Transportation (DOT) and Occupational Safety and Health Administration (OSHA) regulations.
- The labeling must list contents, concentrations, hazard classifications, safety precautions, and the manufacturer or supplier.
- Do not remove or damage manufacturer applied labels, decals or cylinder content information.
- Be sure to contact the manufacturer if you are unsure of a cylinder's contents.
Additional labeling requirements are described in the Compressed Gas Cylinders Safety Guidelines.
When using gas cylinders, wear closed-toe shoes and safety glasses. Gloves may also be required, depending on the chemical or physical hazards of the gas.
Additional personal protective equipment (PPE) and/or clothing requirements may apply depending on the location and activity. Refer to the Compressed Gas Cylinders Safety Guidelines for more information.
Compressed gas cylinders must be purchased through the preferred supplier, Linde (formerly known as Praxair), to ensure the supplier has a cylinder return authorization program.
All toxic and highly toxic gases are required to be purchased through Linde, unless unavailable.
Once the new gas cylinders are ordered, the requestor must add the cylinders to their MyChem inventory along with the safety data sheet (SDS) for the gas.
Ordering information is provided on the UW Procurement website.
If the maximum allowable quantity for a control zone is exceeded, the PI/Shop Manager/Responsible Person must apply for a hazardous materials permit from the local fire department. Information on quantities and limits for maximum allowable quantities can be viewed in MyChem.
Refer to the Compressed Gas Cylinders Safety Guidelines for more information on maximum allowable quantities.
Pressure regulators lower the gas pressure to a usable level. There are two kinds of pressure regulator designs, which appear similar: single-stage and two-stage.
- Single-stage regulators are used when precise control of delivery pressure is not required.
- Two-stage regulators give precise control.
Keep regulators clean and free of surface oil and grease, especially oxidizing gases.
Always use the proper regulator for the gas in the cylinder. Connection fittings, stamped Compressed Gas Association (CGA) numbers, plaques and/or decals on the regulator indicate for which gas the regulator is designed.
Do not use Teflon® tape, putty or other such materials on the threads unless specifically required (or applied) by the manufacturer or vendor.
Signage contains important hazard information regarding the gases stored and must be prominently posted in cylinder storage areas.
- A Caution Sign is required to be posted at entrances to laboratories, shops, and maker spaces where hazardous materials are used and/or stored.
- The Fire Code requires rooms or cabinets containing compressed gases to be conspicuously labeled “COMPRESSED GAS.”
The Caution Sign generated in MyChem can be used to meet both of these requirements.
Additional signage may be required based on the cylinder’s contents and related hazards.
Standard operating procedures (SOPs) for using compressed gases must include possible incident scenarios with appropriate responses and must consider the following factors:
- The nature of the operation (e.g., experimental design, equipment used and type of injury that could occur)
- The potential location of a release or spill (e.g., outdoors versus indoors, in a laboratory, corridor or storage area, on a table, in a hood, or on the floor)
- The quantities of material that might be released and the type of containment (i.e., compressed gas tank size, manifold systems, etc.)
- The chemical and physical properties of the compressed gas (e.g., its physical state, vapor pressure and air or water reactivity)
- The hazardous properties of the compressed gas (e.g., its toxicity, corrosivity and flammability)
- The availability and locations of emergency supplies and equipment
The SOP includes an emergency response plan that identifies building evacuation routes, emergency telephone numbers, chemical containment procedures, fire extinguisher usage, etc.
Visit the Chemical SOPs page to download SOP templates, including the Compressed Gas SOP.
All gas cylinders must be firmly secured to a wall with two chains or straps at 1/3 and 2/3 height of the cylinder to prevent toppling due to seismic hazards.Refer to the article Safely store compressed gases and cryogens for information on safe storage.
Cylinders must be in good condition with an operable valve, and capped when not in use. Locate cylinders in a well-ventilated area at least 20 feet apart from incompatible chemicals. All cylinders must be properly labeled.
Review additional storage requirements in the Compressed Gas Cylinders Safety Guidelines.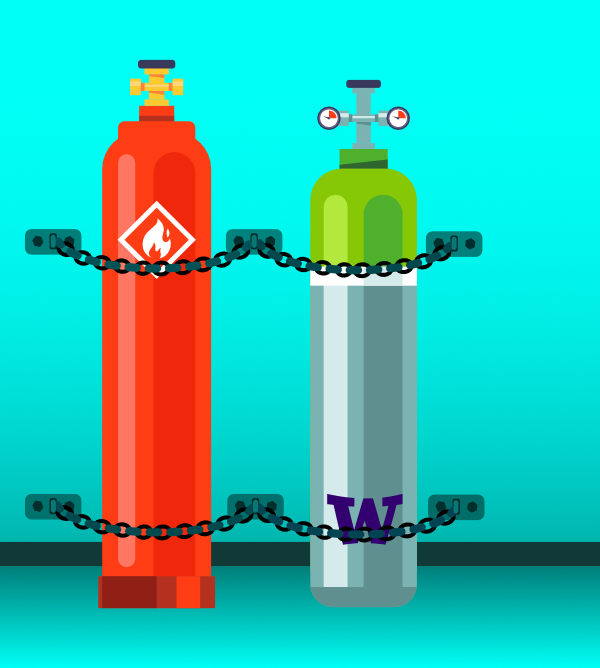
EH&S recommends compressed gas safety training is completed by individuals working with compressed gases prior to handling cylinders.
EH&S offers the following online training courses:
- Compressed Gas Safety for Laboratory Environments
- Compressed Gas Safety (for non-researchers)
- Liquid Nitrogen Safety (required for University personnel working with or around liquid nitrogen)
Gas cylinders and Dewars are particularly vulnerable to damage during transport. Refer to the Gas and Cryogen Transport Focus Sheet for best practices for transporting these materials safely.
Compressed gas cylinders must be handled with caution at all times. Assume all cylinders contain gas under pressure. Treat all gases as hazardous chemicals.
- Read the safety data sheet before you begin using the gas.
- Use cylinders in a well-ventilated area. If you need to use a gas cylinder in spaces with inadequate ventilation, call EH&S at 206.543.7388 to conduct a hazard assessment. Spaces with inadequate ventilation may need oxygen alarms or ventilation failure alarms.
- When turning off the cylinder, turn the gas supply off at the cylinder valve first, depressurize the system, and turn off the regulator.
- Do not use or allow contact with oil or grease on cylinders or their valves.
- Do not empty gas cylinders to a pressure lower than 25 psi (172 kPa). At lower pressures, suction and backflow can cause contamination of residual contents with air if the valve is open.
- Do not use Teflon® tape on cylinder or tube fitting connections, which have metal-to-metal face seals or gasket seals.
Refer to the Compressed Gas Cylinders Safety Guidelines for more information.
Frequently asked questions
Cylinder manufacturers include engraved markings on cylinders to allow for identification of substances in the container; however, do not attempt to identify the compressed gas in the cylinder on your own. Contact the manufacturer if you are unsure of its contents.
Gases and cryogenic liquids can exhibit a number of hazards, and users should be aware of all the hazards associated with the materials they are using, including physical and health hazards that can be associated with compressed gases and cryogens. These hazards are in addition to those due to compression, which can make even inert materials hazardous when compressed.
- Health hazards may include corrosivity, toxicity, and high toxicity.
- Physical hazards may include flammability, oxidization, pyrophoricity, and instability/reactivity.
Check the manufacturer’s safety data sheet and the label on the cylinder to help determine applicable hazards.
- The high pressure in cylinders (4.4 to 6,000 psig) makes the gas cylinder a potential physical explosive rocket that could punch through walls.
- Some gases may be corrosive which could result in change in tissue and/or equipment at the point of contact.
- Cryogenic gases have dangers of low temperature, potential frostbite, and they may expand into large volumes of gas that could displace oxygen and result in suffocation.
- Inert gases and oxidizing reactions may create oxygen deficiency hazards by displacing oxygen and may lead to suffocation. The early symptoms may be dizziness and weakness, which may lead to unconsciousness and death. This is also termed asphyxiation.
- Flammability is a concern especially with the gases acetylene, hydrogen, and propane.
- The permissible exposure limits for toxic materials is very low and so even small exposure is considered to be poisonous.
- Oxygen leaks may create oxygen enriched atmospheres which increase the risk of fire and explosions. Watch a video demonstrating the hazard.
- Cryogenic liquids and dry ice possess the hazard of extreme cold which can cause damage to exposed skin.
- Depending on concentration, mixtures of hazardous gases with those that are inert may or may not present the same hazards.
Refer to the Compressed Gas Cylinders Safety Guidelines for detailed information on hazards, safe use, storage, transport, disposal, leaks and emergencies.
More Information
NFPA 55 - Compressed gas and Cryogenic Fluids Code
WAC: Permissible Exposure Limits for Airborne Contaminants.
OSHA: Compressed Gases
Department of Transportation
ASTM D-323-72
ASTM E681-85
Definitions
The class of gases that are termed compressed gases are non-liquefied gases. This means that they do not become liquid at normal temperatures, even at high pressure. They are either:
- A gas or mixture of gases in a container having an absolute pressure exceeding 40 pounds per square inch (psi) at 70° F (21° C);
- A gas or mixture of gases in a container having an absolute pressure exceeding 104 psi at 130° F regardless of the pressure at 70° F(21° C); or
- A liquid having a vapor pressure exceeding 40 psi at 100° F (38° C); as determined by ASTM D-323-72.
Examples include: helium, nitrogen, oxygen, and argon.
A chemical that causes visible destruction of, or irreversible alterations in, living tissue by chemical action at the point of contact. Corrosive gases can also corrode valves and piping, making a release more likely if not properly managed.
This class of gases that are referred to as dissolved gases are those that are dissolved in another substance.
Acetylene is the only commonly used dissolved gas and it is dissolved in acetone. It is chemically unstable and flammable.

A cryogenic fluid that is flammable in its vapor state
A material which is ignitable at NTP when in a mixture of 13 percent or less by volume with air; or has flammable range with air of at least 12 percent, regardless of lower limit
A liquefied compressed gas which, under a charged pressure is partially liquid at NTP and which is flammable
A material which produces a lethal dose or lethal concentration (LD50) in air of 200 parts per million by volume or less of gas or vapor, or 2 mg per liter or less of mist fume or dust, when administered by continuous inhalation for one hour (or less if death occurs within 1 hour) to albino rats weighing between 200 - 300 grams each
The class of gases that are referred to as liquefied compressed gases become liquid at normal temperatures when they are pressurized inside a gas cylinder.
Examples of liquefied gases include: ammonia, chlorine, methane, natural gas and liquefied petroleum gas or liquid petroleum gas (lpg) which are propane and butane.

Non-pressurized containers are typically open-topped vessels or Dewars with loose fitting covers to minimize evaporation or off gassing and range from one to 50 liters in size.
68° F (20° C) and 14.7 psi
A cryogenic fluid with oxidizing properties which increase the burning rate of normal combustibles
A gas that can support and accelerate combustion of other materials more than air does
Pressurized containers have vents, dispensing hoses, and pressure gauges. Pressure relief valves are spring loaded devices set at a specific pressure that relieve excessive pressure, reclose and reseal to prevent further release of product.
A chemical with an auto-ignition temperature in air, at or below a temperature of 130°F (54°C)
The class of gases that are referred to as refrigerated liquefied gases are also known as cryogens. They are:
- Kept at very low temperatures.
- Extremely cold with boiling points below -150° C.
- Heavier than air under cold temperature conditions and can accumulate near the floor.
- Able to expand into very large volumes of gas from small amounts of refrigerated liquefied gas liquid.
Examples of refrigerated liquefied gases (cryogens) include: helium, liquid nitrogen, and liquid argon. Carbon dioxide and nitrous oxide, which have slightly higher boiling points, are also included in this category.
Bulk storage and container transport of cryogenic gases is in liquid, double-walled, vacuum-sealed containers that can be either pressurized or non-pressurized.


A chemical that has a median lethal dose (LD50) in air of more than 200 parts per million by volume of gas or vapor or more than 2 mg per L but not more than 20 mg per liter of mist, fume or dust, when administered by continuous inhalation for 1 hour (or less if death occurs within 1 hour) to albino rats weighing between 200-300 grams each.
A material other than an explosive, which in the pure state or as commercially produced, will vigorously polymerize, decompose, condense or become self-reactive and undergo other violent chemical changes, including explosion, when exposed to heat, friction or shock, or in the absence of an inhibitor, or in the presence of contaminants, or in contact with incompatible materials